
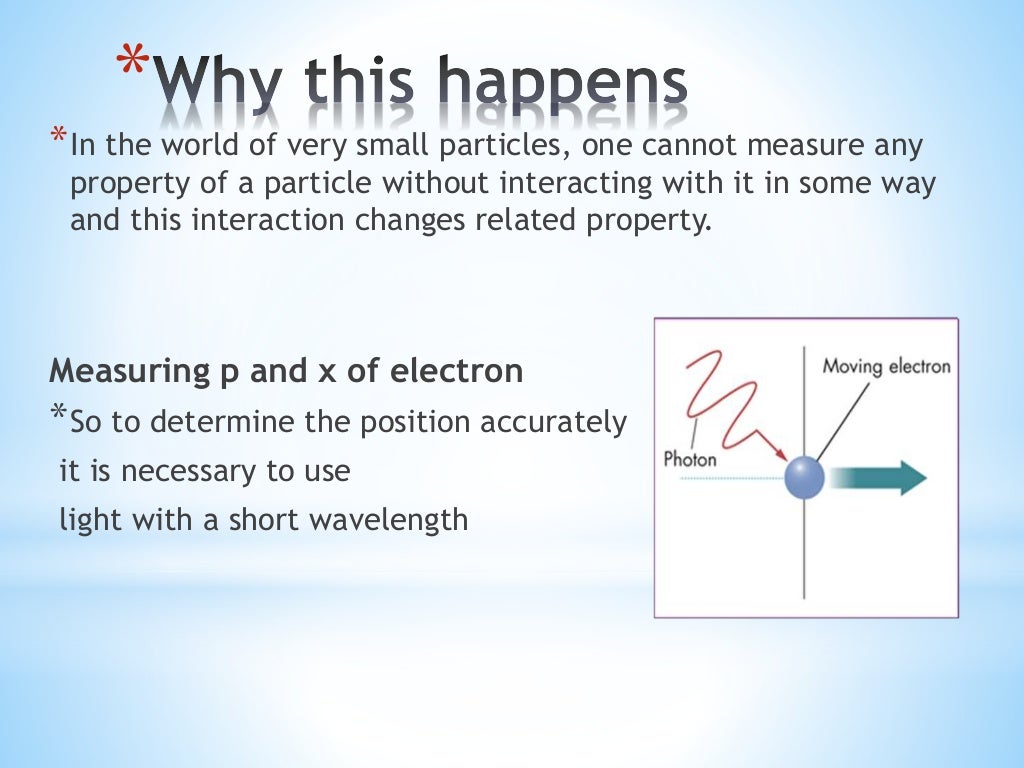
The HUP doesn't say anything in particular about any single measurement of position or momentum of a particle. One interpretation of this is that if you prepare a state with a well-defined momentum, then that state will have a relatively large spread of position measurements and, vice versa. Where #\sigma_x, \sigma_p# are the standard deviations for position and momentum respectively. p y h / w so the act of measuring the electron’s y position has fuzzed out its y momentum by precisely the amount required by the uncertainty principle. German physicist and Nobel Prize winner Werner Heisenberg created the famous uncertainty principle in 1927, stating that we cannot know both the position. This is helped by the sense of progression, which allows for some impressive environment designs. If you then take the standard deviation of these measurements, then they obey: Putting in p x h /, we find immediately that. Uncertainty Principle absolutely knocks it out of the park when it comes to its visuals and tone. There is a closely related principle in Fourier analysis that. The product of the uncertainties in the two quantities has a lower bound. If you prepare a large number of particles and measure, say, the momentum (at some time #t#) for half of them and the position (at time #t#) for the other half of them, then you will get a spread of measurements for both momentum and position. Heisenberg’s uncertainty principle says there is a limit to how well you can know both the position and momentum of anything at the same time.

Werner Heisenberg, a German physicist, determined that our observations have an effect on the behavior of. Similar uncertainty principles hold for non-quantum mechanical. This is called Heisenbergs Uncertainty Principle. It applies to the measurements of, for example, momentum and position on an ensemble of identically prepared particles. As defined in quantum mechanics, it is also called Heisenbergs uncertainty principle. The Heisenberg Uncertainty Principle (HUP) is a statistical law. If velocity is delta position vs delta time and you know the velocity and change in time exactly why is it impossible to find the exact position of the electron? Same question for energy and position.


 0 kommentar(er)
0 kommentar(er)
